The free energy of mixing is defined as:
ΔGmix = ΔHmix – TΔSmix
The variation in free energy as a function of composition and temperature can be considered for three different situations: an ideal solution, a non-ideal solution with a positive enthalpy of mixing, and a non-ideal solution with a negative enthalpy of mixing.
1. Ideal solid solution, ΔHmix = 0
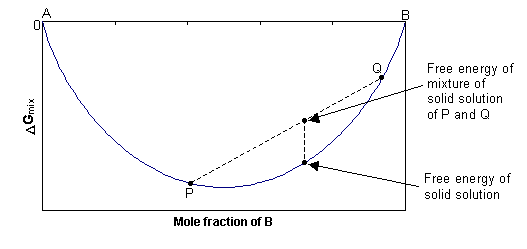
In this case, ΔGmix = –TΔSmix, and since ΔSmix is always positive, ΔGmix is always negative. At any composition, the free energy of the single-phase solid will be lower than the combined free energy of any mixture of the two separate phases, as shown in the diagram below. The solid solution is stable as a single phase, with disordered cation distribution at all compositions and temperatures.
2. Non-ideal solid solution, ΔHmix > 0
At high temperatures, the –TΔSmix term dominates, and the free energy curve resembles that of the ideal solution. As the temperature decreases, the ΔHmix term and the –TΔSmix term become similar in magnitude and the resulting free energy curve shows two minima and a central maximum.
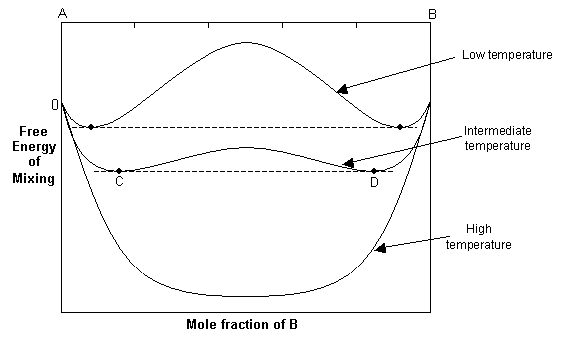
The common tangent rule can be used to determine the equilibrium state of the solid solution. The common tangent will touch the free energy curve at C and D, and for bulk compositions between these points, the free energy of the single-phase solution is higher than that of a mixture of C and D. Hence at equilibrium, the system will minimise its free energy by exsolving to two phases with compositions C and D. For compositions outside C and D, the solid solution will still be stable as a single phase since it has a lower free energy than a mixture of two phases.
3. Non-ideal solid solution, ΔHmix < 0
In this case, there is a strong driving force for ordering when the A:B ratio is about 1:1. The fully-ordered phase has zero configurational entropy, because there are only two ways to arrange the atoms: the ordered and anti-ordered states (which are equivalent). However, this state has a low enthalpy, due to the energetically-favourable arrangement of ions, which stabilises the ordered phase at low temperatures. In contrast, the fully disordered solid solution has a high configurational entropy, which stabilises it at high temperatures. At a certain critical temperature, there will be a phase transition from the ordered to the disordered phase.
Leave a Reply