Electrochemical systems are not only essential for our society but are also common in everyday life. Imagine a world without batteries to power your personal electronic devices. How would travel change without low-cost aluminum that is essential for aircraft? What if corrosion of the steel in bridges, the hulls of ships, superstructures of buildings, and pipelines couldn’t be controlled? These examples, electrochemical energy storage, industrial electrolysis, and corrosion, are some of the major applications of electrochemical engineering. Following development of the fundamental principles of electrochemical engineering, these and other applications are covered in separate chapters.
We all know that the storage of energy using batteries (Chapters 7 and 8) is an essential feature for countless technologies. The size of the global battery market is more than 50 billion dollars annually. Applications of batteries are widespread in society, ranging from basic consumer electronics, to automobiles, implanted medical devices, and space travel. Energy storage is needed where portability is required, emergency power is desired, or simply when electrical demand and supply are not matched. Batteries store energy chemically and convert that energy to electricity as needed. For many applications, the conversion between chemical and electrical energy must be extremely efficient and, in some cases, nearly reversible in order for batteries to be practical. Electrochemical double-layer capacitors (Chapter 11) can also be used to store energy.
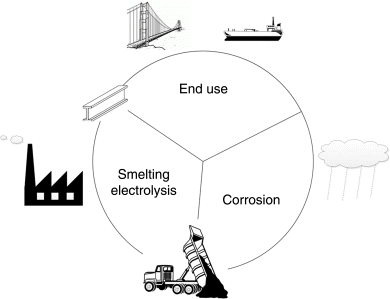
The purpose of industrial electrolysis is to use electrical energy to convert raw materials into desired products. This transformation of raw materials takes place in an electrochemical reactor. Industrial electrolytic processes (Chapter 14) consume about of 6% of the U.S. electricity supply. Although a number of metals and other materials can be produced electrochemically, the main products of electrolysis are aluminum, chlorine, and sodium hydroxide (caustic soda). Low-cost electricity and electrochemical routes for production transformed aluminum from an expensive and rarely used metal to a commodity material that is essential for society. Chlorine and sodium hydroxide, produced simultaneously on a huge scale by electrolysis, are required for the production of a variety of materials, including plastics, paper, soaps, solvents, and a large number of other chemicals.
The many desirable properties of metals have led to their widespread use in industry and, frankly, in nearly every aspect of our lives. Corrosion (Chapter 16) is the unwanted attack of metals by their environment. The manufacture of metals is sometimes done electrochemically as described above for the production of aluminum. We can think of corrosion as the reverse process, by which metals are converted back into their oxide form. This cycle is illustrated in Figure 1.2. The process of corrosion is electrochemical in nature. The global economic cost of corrosion has been estimated to be several trillion dollars annually. Therefore, it is important that engineers understand the conditions under which corrosion is likely to occur. They should also be able to measure, predict, and mitigate the negative impacts of corrosion. Avoidance of corrosion is incorporated into the appliances found in our homes. Additionally, corrosion prevention is essential for maintaining the integrity of the bridges over which we travel, and an integral part of the design of natural gas and oil pipelines that supply our energy. These are just a few of the areas where understanding corrosion is important.
Electrodeposition is the electrochemical deposition of a metal onto a surface (Chapter 13). Electrodeposition is used for decoration, to provide protective coatings, and is essential in the fabrication of interconnects for large-scale integrated circuits. Most metal fasteners (screws, nails, clips, and bolts to name but a few) are coated for appearance, to improve their corrosion resistance, or to reduce friction. Many of these fasteners use electrochemical processes to provide these coatings.
Roughly 10% of the world’s population is afflicted with diabetes. Since treatment requires regular monitoring of blood glucose levels, the measurement of glucose concentration is one of the most frequent tests conducted. Did you know that most of the billions of these tests are done electrochemically? These sensors work by measuring the current generated from electrochemical oxidation of glucose. Modern automobiles use oxygen sensors to allow efficient operation of the fuel injection system. These sensors are also electrochemical devices.
We hope that this section has helped you gain an increased appreciation of the importance of electrochemical systems. In addition to the applications noted above, there are other promising technologies in development. These include fuel cells (Chapters 9 and 10), which convert clean fuels into energy at efficiencies much higher than that of a combustion engine. Fuel cells have been essential for manned space flight, but have yet to achieve widespread commercial success. Semiconductor electrochemistry (Chapter 15) offers opportunities for photoelectrochemical processes to convert sunlight into electricity or to produce useful chemicals through artificial photosynthesis. The list of developing applications extends well beyond these two examples, and electrochemistry will undoubtedly continue to provide new products and processes that will improve our quality of life and benefit humankind.
Before we can examine applications in detail, some fundamental principles must be mastered. These fundamental principles provide the foundation for all of the applications mentioned above. We begin by introducing some basic terminology, as well as reviewing scientific units and the conventions adopted in this text.
Leave a Reply