Wilson2 made a bold assumption regarding the temperature dependence of Ωij. Wilson’s original parameter used in the literature is Λji, but it is related to Ωij in a very direct way. Wilson assumes3
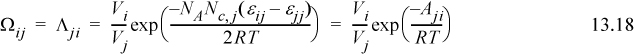
(note: Λii = Λjj = 1, and Aij ≠ Aji even though εij = εji), and integration with respect to T becomes very simple. Assuming Nc,j = 2 for all j at all ρ,
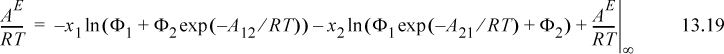
A convenient simplifying assumption before proceeding further is that GE ~ AE. This corresponds to neglecting the excess volume of mixing relative to the other contributions and is quite acceptable for liquids. The customary way of interpreting GE/RT is to separate it into an energetic part known as the residual contribution, (GE/RT)RES, that vanishes at infinite temperature or when ε12 – ε22 = 0 and ε21 – ε11 = 0, and a size/shape part known as the combinatorial contribution, (GE/RT)COMB, that represents the infinite temperature limit at the liquid density. For Wilson’s equation, the first two terms vanish at high T, so
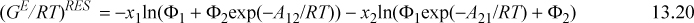
For the combinatorial contribution, Wilson used Flory’s equation,
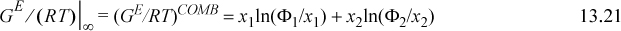
It should be noted that the assumption of the temperature dependence of Eqn. 13.18 has been made for convenience, but there is some justification for it, as we show in Section 13.7. Wilson’s equation becomes
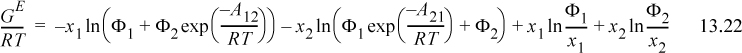
Algebraic rearrangement of Wilson’s equation results in the form that is usually cited,
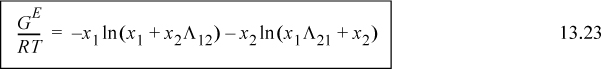
For a binary system, the activity coefficients from the Wilson equation are:
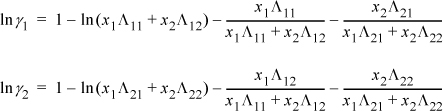
Noting that Λ11 = Λ22 = 1, and looking back at Eqn. 13.6, we can also see that for the first equation ![]() . We can rearrange this expression to obtain the slightly more compact relation:
. We can rearrange this expression to obtain the slightly more compact relation:
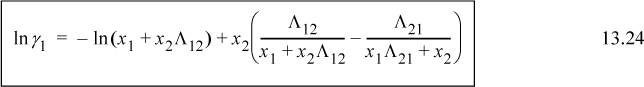
Similar rearrangement of the second expression gives:
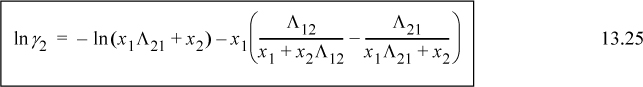
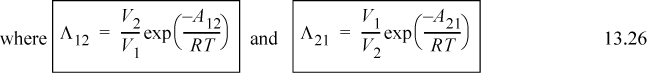
![]() Parameters for the Wilson equation, Aji. Note that the literature values often include energy units. Use the correct R!
Parameters for the Wilson equation, Aji. Note that the literature values often include energy units. Use the correct R!
![]() Wilson’s equation is incapable of representing LLE.
Wilson’s equation is incapable of representing LLE.
One limitation of Wilson’s equation is that it is unable to model liquid-liquid equilibria, but it is reasonably accurate for modeling the liquid phase when correlating the vapor liquid equilibria.
Extending Eqn. 13.23 to a multicomponent solution,
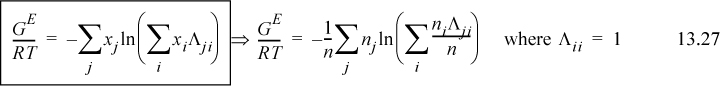
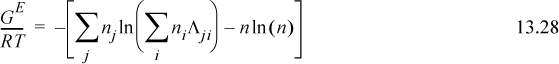
To determine activity coefficients, the excess Gibbs energy is differentiated. Differentiating the last term,
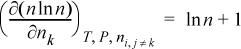
and letting “sum” stand for the summation of Eqn. 13.28, and combining,
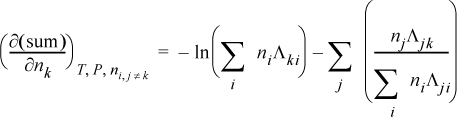
combining,
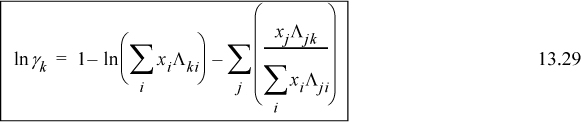
Example 13.3. Application of Wilson’s equation to VLE
For the binary system n-pentanol(1) + n-hexane(2), the Wilson equation constants are A12 = 1718 cal/mol, A21 = 166.6 cal/mol. Assuming the vapor phase to be an ideal gas, determine the composition of the vapor in equilibrium with a liquid containing 20 mole% n-pentanol at 30°C. Also calculate the equilibrium pressure. ![]() ;
; ![]() .
.
Solution
From CRC,
ρ1 = 0.8144 g/ml (1mole/88g) ⇒ V1 = 108 cm3/mole
ρ2 = 0.6603 g/ml (1mole/86g) ⇒ V2 = 130 cm3/mole
Note: ρ1 and ρ2 are functions of T but ρ1/ρ2 ≈ const. ⇒ V2/V1 = 1.205 assumed at all T.
Utilizing Eqn. 13.26,
Λ12 = 1.205 exp(– 1718/1.987/303) = 0.070; Λ21 = 1/1.205 exp(– 166.6/1.987/303) = 0.629
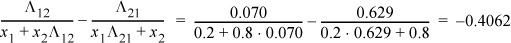
In γ1 = 1.0408 ⇒ γ1 = 2.822; In γ2 = 0.1584 ⇒ γ2 = 1.172
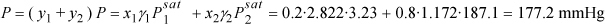
y1 = x1γ1Psat/P = 0.2·2.822·3.23/177.2 = 0.0103
Leave a Reply