An electrochemical reaction is a reaction where the transfer of electrons from a species being oxidized to a species undergoing reduction takes place through an electronic conductor. Typically, that conductor is a metal. Because the electron transfer takes place through a conductor rather than directly between the reacting species, we can separate the two electron-transfer reactions and use the flow of electrons (current) between them to do work. The oxidation or anodic reaction takes place at the anode, and the reduction reaction or cathodic reaction takes place at the cathode. An example of an anodic reaction is
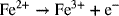
where 1 mol of electrons is produced for every mole of Fe(II) that is oxidized. An example of a cathodic reaction is
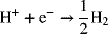
Both an oxidation reaction and a reduction reaction are required to make an electrochemical cell. Therefore, an anodic or a cathodic reaction is referred to as a half-cell reaction as described in Chapter 1. The full-cell reaction is obtained by adding two half-cell reactions. For example, the two reactions above can be added to yield the following full-cell reaction:
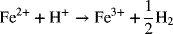
Note that there are no net electrons in the full-cell reaction, and that the charge on both sides of the reaction must balance. Identifying and understanding these half-cell reactions is essential to the analysis of electrochemical systems. Common reactions are tabulated in Appendix A.
Leave a Reply