Now that we have an accounting for the thermodynamics of bond formation, it is natural to wonder what happens to the thermodynamics as the bond energy approaches infinity. This would be a natural limit for covalent bond formation. Having a theoretical basis for nonspherical molecules would be a big step forward, considering that all theories discussed until now have been based on spherical molecules. Of course, we added correction terms like α(T, ω) to the Peng-Robinson model, but this was done with no theoretical basis. Wertheim’s theory provides an opportunity to develop meaningful guidelines for shape effects.
The key step is to find the contribution to the equation of state from forming a bond in the limit of infinite bond energy. The result for binary association, Eqn. 19.54, is convenient to illustrate the key points. At first glance, the limit may not seem obvious, because the X term in Achem must approach zero and the log term would then be undefined. This issue can be resolved by substituting, say, 1 – X = X2Δ. We use Abond to denote the covalent nature of the bonds.
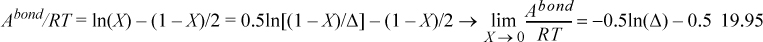
Example 19.6. Complex fugacity for the van der Waals model
A sample calculation with a specific reference equation of state should clarify these results. Let K12AD = K22AD = 0.72 cm3/mol and ε12AD = ε22AD = 20 kJ/mol, b1=27.5 and b2=20.4 cm3/mol.
a. Derive Zchem and ln(φkchem) adapting the definition of Δ from Eqn. 19.44.
b. Evaluate the expressions for trimethylamine(1) + methanol(2) at x1 = 0.5, ρ = 0.0141mol/cm3, and T = 300K.
Solution
a. ΔijAD = ρKAD(exp(βεijAD) – 1)/(1 – ηP) = Δ.
Eqn. 19.65 shows that ηP∂Δ/∂ηP = Δ/(1 – ηP)
Substituting gives Zchem = –0.5h/(1 – ηP). For ln(φkchem) Eqn. 19.93 requires n∂(ΔijAD/n)/∂nk, ![]()
Similarly, n∂(ΔijDA/n)/∂nk = (ΔijDAbkρ)/(1 – η). Substituting this result into Eqn. 19.93 gives,
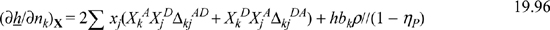
b. Evaluating these expressions, Δ11DA = Δ12DA= 0 because trimethylamine (TMA) has no donors, so X1D = 1. It may seem odd to represent X1D = 1 when there are no donors, but site occupation is impossible when Δ = 0 for that site.
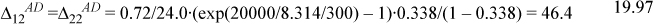


To solve Eqns. 19.93 for X1A, X2A, and X2D,

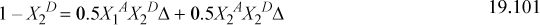

This gives three equations. Rearranging (below) shows that X1A = X2A. We can replace these to obtain a quadratic equation in terms of X2D. Usually, we would need to iterate to solve for X2D.


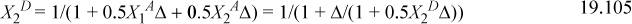
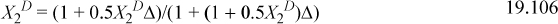
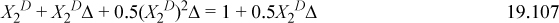
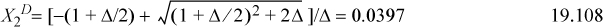
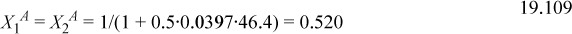
This shows that X2D is almost completely bonded.
By Eqn. 19.88,
h = x12X1AX1DΔ11AD + 2x1x2X1AX2DΔ12AD + x22X2AX2DΔ22AD + x12X1DX1AΔ11DA + 2x1x2X1DX2AΔ12DA + x22X2DX2AΔ22DA
Substituting x1, x2, and X gives
h = 0 + 2·x1x2·X1AX2D·46.4 + x22X2AX2D·46.4 + 0 + 0 + x22X2DX2A·46.4 = 0.960
Zchem = –0.5h/(1 – ηP) = –0.480/(1 – 0.338) = –0.725
By Eqn. 19.92,
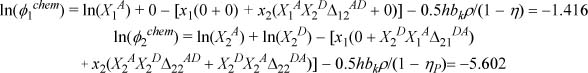
There are several points of interest in this result. The acceptors in this mixture outnumber donors by two to one. Therefore, it is impossible that XiA< 0.5, and, in fact, X2D ~ 2·(X1A – 0.5) because the lack of donor saturation is reflected twice, in X1A and X2A. The compressibility factor is depressed in a simple way that sums over all donors and acceptors, but the fugacity is depressed more for the alcohol than for the amine. There are three ways for the alcohol to interact, but only one for the amine, so the depression of the fugacity is much greater. On the other hand, the fugacity of the alcohol is depressed less in the mixture than in the pure fluid because relatively fewer acceptors are bonded (ln(φ2chem)= –6.105 at x2 = 1). So the activity of the alcohol in the mixture is relatively enhanced by hydrogen bonding while the activity of the amine is depressed at all compositions.
Example 19.7. More complex fugacity for the van der Waals model
Evaluate the expressions for Zchem and ln(φkchem) of trimethylamine(1) + methanol(2) at x1 = 0.4, ρ = 0.0141 mol/cm3, and T = 300 K. Let K12AD = K22AD = 0.72 cm3/mol and 1.25ε12AD = ε22AD = 20 kJ/mol, b1 = 27.5, and b2 = 20.4 cm3/mol.
Solution
The difference between this example and the previous is that ε12AD ≠ ε22AD, indicating that the solvation is slightly weaker than the alcohol association. Because of this lack of symmetry, an iterative solution for X is required. Recalling part (a) of the previous example,
Zchem = –0.5h/(1 – ηP)
For ln(φkchem): –∑ xj(XkA XjDΔkjAD + XkDXjA ΔkjDA) – 0.5hbkρ/(1 – ηP)
Substituting the mole fractions and solving for Δ’s,
b = 0.4·27.5 + 0.6·20.4 = 23.4; ηP = 0.0141·23.4 = 0.328. This is slightly less than Eqn 19.99.
Δ22AD = ρKAD(exp(βε22AD) – 1)/(1 – η) = 45.8. Δ12AD = 9.21; Δ11DA = Δ12DA = 0.
1 – X1A = 0.5X1AX2DΔ12AD; 1 – X2D = 0.5X1AX2DΔ12AD + 0.5X2AX2DΔ22AD;
1 – X2A = 0.5X2AX2DΔ22AD;
X1A = 1/(1 + 0.4X2DΔ12AD); X2A = 1/(1 + 0.6X2DΔ22AD);
X2D = 1/(1 + 0.4X1AΔ12AD + 0.6X2AΔ22AD);
Unlike the previous example, an explicit solution is not found. The previous example was contrived to achieve an exact solution, but this is rarely possible. Normally, we must iterate to achieve a numerical solution. It is convenient to guess X2D, then compute X1A and X2A, then check the new value of X2D based on X1A and X2A. To initialize X2D, a reasonable estimate can be based on a variation of the solution for ΔkjAD =(ΔkkADΔjjAD)1/2.
Applying Eqn. 19.94,
(–1 + 1/X2D) ≈ Σ xjΔ2jAD/[1 + (ΔjjAD)1/2] = 0.122 ⇒ X1A = 0.597; X2A = 0.230; X2D = 0.105;
Five more iterations give, X1A = 0.678; X2A = 0.297; X2D = 0.0855. Five iterations is actually a large number because this particular mixture deviates substantially from the SRCR.
X1A = 1/(1 + 0.4·0.0857·9.21)= 0.678
X2A = 1/(1 + 0.6·0.0857·45.8)= 0.297
X2D = 1/(1 + 0.4·0.678·9.21 + 0.6·0.297·45.8) = 0.0855
Substituting into Eqn. 19.88
h = x12X1AX1DΔ11AD + 2x1x2X1AX2DΔ12AD + x22X2AX2DΔ22AD
+ x12X1DX1AΔ11DA + 2x1x2X1DX2AΔ12DA + x22X2DX2AΔ22DA
h = 0 + 2·x1x2·X1AX2D·9.21 + x22X2AX2D·45.8 + 0 + 0 + x22X2DX2A·45.8 = 1.097
Zchem = –0.5h/(1 – ηP) = –0.480/(1 – 0.328) = –0.816
ln(φ1chem) = ln(X1A) – [x1(0 + 0) + x2(X1AX2DΔ12AD + 0)] – 0.5hbkρ/(1 – ηP) = –2.228
ln(φ2chem) = ln(X2A) + ln(X2D) – [x1(0 + X2DX1AΔ21DA)
+ x2(X2AX2DΔ22AD + X2DX2AΔ22DA)] – 0.5hbkρ/(1 – ηP) = –5.855
These results show that a 20% change in ε12AD compared to Example 19.6 gives a 500% change in Δ12AD. That is fairly sensitive. This change in Δ12AD is primarily responsible for the increase in X1A from 0.520 to 0.679 and the decrease of X2A from 0.520 to 0.298. Overall, the chemical contributions are slightly stronger because the composition of amine has been reduced.
Eqn. 19.95 is helpful when Δ→∞ because Z can be obtained by differentiation of A. Taking the derivative,
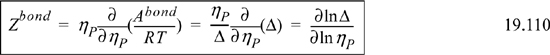
From a model for Δ, the bonding contribution to the EOS results. For example, if Δ is given by the van der Waals model,
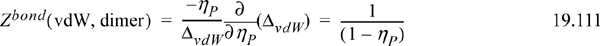
Generalizing this result to a chain with m segments, there are (m–1) bonds per chain. For example, continuing with the vdW model,
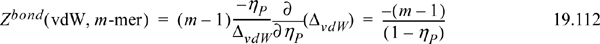
This is essentially Wertheim’s theory of polymerization, although Wertheim specifically treated the case resulting in a mixture with a range of molecular weights and average degree of polymerization of <m>.22
Leave a Reply