We now focus on the second summation of Eqn. 17.12. The ratio appearing in the logarithm is known as the activity, (cf. Eqns. 11.23 for a liquid, but now in a general sense):

The numerator ![]() represents a mixture property that changes with composition. We have developed methods to calculate
represents a mixture property that changes with composition. We have developed methods to calculate ![]() in Eqns. 10.61 (ideal gases), 10.68 (ideal solutions), 11.14 (real solution using γi), Eqn. 15.13 (real gases using
in Eqns. 10.61 (ideal gases), 10.68 (ideal solutions), 11.14 (real solution using γi), Eqn. 15.13 (real gases using ![]() ). The denominator represents the component at a specific standard state, which includes specification of a fixed composition (which can be pure or a mixture state).
). The denominator represents the component at a specific standard state, which includes specification of a fixed composition (which can be pure or a mixture state).
The second sum of Eqn. 17.12 can be manipulated after inserting the activity notation,

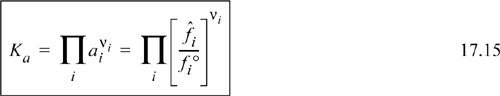
In a reacting mixture ![]() and/or ai will change as the reacting composition moves toward equilibrium. However, at equilibrium, the product term of activities is extremely important. We define the product term at equilibrium as the equilibrium constant Ka with the a subscript to denote that activity is used:
and/or ai will change as the reacting composition moves toward equilibrium. However, at equilibrium, the product term of activities is extremely important. We define the product term at equilibrium as the equilibrium constant Ka with the a subscript to denote that activity is used:
![]() General equilibrium constraint.
General equilibrium constraint.
Combining the definition of the equilibrium constant with Eqn. 17.12, the first summation can be used to find the value of the constant:

Note that use of the term constant can be misleading because it depends on temperature. It is constant with respect to feed composition and changing mole numbers of reacting species as we will show below. We use a subscript a on the equilibrium constant to stress that it depends on activities. As we show later, there are other approximations for the equilibrium constant, and subscripts are used to differentiate between different conventions.
The Equilibrium Constant for Ideal Gases
The activity is a general property defined by Eqn. 17.13. We have seen it applied to liquids in Section 11.5. For ideal gases, the numerator of the activity is ![]() . We complete the formula for activity by selecting the standard state. For gaseous reacting species, the convention is to use a standard state of the pure gas at P° = 1 bar. For an ideal gas, fio = Po (Eqn. 9.29). Thus, fio = 1 bar. The fugacity ratio (activity) is dimensionless provided that we always express the partial pressure in bar. The second sum of Eqn. 17.12 for ideal gases simplifies to
. We complete the formula for activity by selecting the standard state. For gaseous reacting species, the convention is to use a standard state of the pure gas at P° = 1 bar. For an ideal gas, fio = Po (Eqn. 9.29). Thus, fio = 1 bar. The fugacity ratio (activity) is dimensionless provided that we always express the partial pressure in bar. The second sum of Eqn. 17.12 for ideal gases simplifies to

where the first two equalities are general, but the last is restricted to ideal gases. We will later reevaluate the fugacity ratio for nonideal gases, liquids, and solids. Now let us examine the first sum of Eqn. 17.12 which will give us the value of Ka.
Leave a Reply