Equilibrium constants in electrolyte literature are often presented on the molal scale. For clarity in this section, we will use Ka,m to denote the molality equilibrium constant and Ka to denote the rational (Henry’s law scale) using mole fractions. Recall that the solvent (usually water) is on the Lewis-Randall scale. Using the molality scale for the electrolytes,

On the rational (Henry’s law) mole fraction scale, we have

To convert, consider the ln of each equation and take the difference. Inserting Eqn. 18.170,
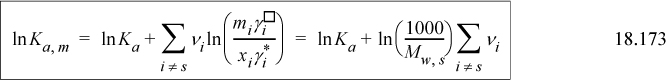
For a 1-1 electrolyte such as NaCl, ![]() , thus
, thus ![]() . If a Ka is desired on the Lewis-Randall scale, similar conversions can be done using infinite dilution activity coefficients.
. If a Ka is desired on the Lewis-Randall scale, similar conversions can be done using infinite dilution activity coefficients.
Leave a Reply