P17.1. An equimolar mixture of H2 and CO is obtained by the reaction of steam with coal. The product mixture is known as “water-gas.” To enhance the H2 content, steam is mixed with water-gas and passed over a catalyst at 550°C and 1 bar so as to convert CO to CO2 by the reaction:
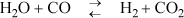
Any unreacted H2O is subsequently condensed and the CO2 is subsequently absorbed to give a final product that is mostly H2. This operation is called the water-gas shift reaction. Compute the equilibrium compositions at 550°C based on an equimolar feed of H2, CO, and H2O.
Data for 550°C:

P17.2. One method for the production of hydrogen cyanide is by the gas-phase nitrogenation of acetylene according to the reaction: N2 + C2H2 ![]() 2HCN. The feed to a reactor in which the above reaction takes place contains gaseous N2 and C2H2 in their stoichiometric proportions. The reaction temperature is controlled at 300°C. Estimate the product composition if the reactor pressure is: (a) 1 bar; (b) 200 bar. At 300°C,
2HCN. The feed to a reactor in which the above reaction takes place contains gaseous N2 and C2H2 in their stoichiometric proportions. The reaction temperature is controlled at 300°C. Estimate the product composition if the reactor pressure is: (a) 1 bar; (b) 200 bar. At 300°C, ![]() .
.
P17.3. Butadiene can be prepared by the gas-phase catalytic dehydrogenation of 1-butene: C4H8 ![]() C4H6 + H2. In order to suppress side reactions, the butene is diluted with steam before it passes into the reactor.
C4H6 + H2. In order to suppress side reactions, the butene is diluted with steam before it passes into the reactor.
a. Estimate the temperature at which the reactor must be operated in order to convert 30% of the 1-butene to 1,3-butadiene at a reactor pressure of 2 bar from a feed consisting of 12 mol of steam per mole of 1-butene.
b. If the initial mixture consists of 50 mol% steam and 50 mol% 1-butene, how will the required temperature be affected?
P17.4. Ethylene oxide is an important organic intermediate in the chemical industry. The standard Gibbs energy change at 298 K for the reaction ![]() mole. This large negative value of ΔGoT indicates that equilibrium is far to the right at 298 K. However, the direct oxidation of ethylene must be promoted by a catalyst selective to this reaction to prevent the complete combustion of ethylene to carbon dioxide and water. Even with such a catalyst, it is thought that the reaction will have to be carried out at a temperature of about 550 K in order to obtain a reasonable reaction rate. Since the reaction is exothermic, an increase in temperature will have an adverse effect on the equilibrium. Is the reaction feasible (from an equilibrium standpoint) at 550 K, assuming that a suitable catalyst selective for this reaction is available? For ethylene oxide, ΔHf298 = -52.63 kJ/mol. Heat capacity equations (in J/mole-K) for the temperature range involved may be approximated by CP,C2H4O = 6.57 + 0.1389 T(K); CP,C2H4 = 15.40 + 0.0937 T(K); CP,O2 = 26.65 + 0.0084 T(K)
mole. This large negative value of ΔGoT indicates that equilibrium is far to the right at 298 K. However, the direct oxidation of ethylene must be promoted by a catalyst selective to this reaction to prevent the complete combustion of ethylene to carbon dioxide and water. Even with such a catalyst, it is thought that the reaction will have to be carried out at a temperature of about 550 K in order to obtain a reasonable reaction rate. Since the reaction is exothermic, an increase in temperature will have an adverse effect on the equilibrium. Is the reaction feasible (from an equilibrium standpoint) at 550 K, assuming that a suitable catalyst selective for this reaction is available? For ethylene oxide, ΔHf298 = -52.63 kJ/mol. Heat capacity equations (in J/mole-K) for the temperature range involved may be approximated by CP,C2H4O = 6.57 + 0.1389 T(K); CP,C2H4 = 15.40 + 0.0937 T(K); CP,O2 = 26.65 + 0.0084 T(K)
P17.5. The water-gas shift reaction is to be carried out at a specified temperature and pressure employing a feed containing only carbon monoxide and steam. Show that the maximum equilibrium mole fraction of hydrogen in the product stream results when the feed contains CO and H2O in their stoichiometric proportions. Assume ideal gas behavior.
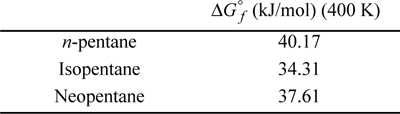
P17.6. Assuming ideal gas behavior, estimate the equilibrium composition at 400 K and 1 bar of a reactive gaseous mixture containing the three isomers of pentane. Standard formation data at 400 K are
P17.7. One method for the manufacture of synthesis gas depends on the vapor-phase catalytic reaction of methane with steam according to the equation ![]() . The only other reaction which ordinarily occurs to an appreciable extent is the water-gas shift reaction. Gibbs energies and enthalpies for the problem are tabulated below in kJ/mol.
. The only other reaction which ordinarily occurs to an appreciable extent is the water-gas shift reaction. Gibbs energies and enthalpies for the problem are tabulated below in kJ/mol.
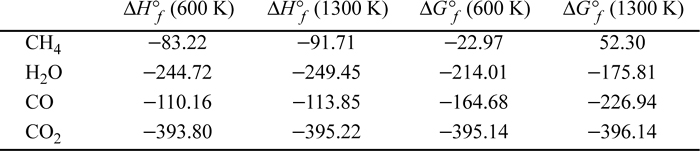
Compute the equilibrium compositions based on a 1:1 feed ratio at 600 K and 1300 K and 1 bar and 100 bars.
P17.8. Is there any danger that solid carbon will form at 550°C and 1 bar by the reaction 2CO = Cs + CO2? (ANS. Yes)
P17.9. Calculate the equilibrium percent conversion of ethylene oxide to ethylene glycol at 298 K and 1 bar if the initial molar ratio of ethylene oxide to water is 3.0.
C2H4O(g) + H2O(l) = (CH2OH)2 (1 M aq solution) ![]()
To simplify the calculations, assume that the gas phase is an ideal gas mixture, that γw = 1.0, and that the shortcut K value is applicable for ethylene oxide and ethylene glycol.
P17.10. Acetic acid vapor dimerizes according to 2A1 ![]() A2. Assume that no higher-order associations occur. Supposing that a value for Ka is available, and that the monomers and dimers behave as an ideal gas, derive an expression for yA1 in terms of P and Ka. Then develop an expression for PV/noRT in terms of yA1, where no is the superficial number of moles neglecting dimerization. Hint: Write no/nT in terms of yA1 where nT = n1 + n2.
A2. Assume that no higher-order associations occur. Supposing that a value for Ka is available, and that the monomers and dimers behave as an ideal gas, derive an expression for yA1 in terms of P and Ka. Then develop an expression for PV/noRT in terms of yA1, where no is the superficial number of moles neglecting dimerization. Hint: Write no/nT in terms of yA1 where nT = n1 + n2.
Leave a Reply