Briefly, an electrolyte is a substance that dissociates into charged species in a liquid phase. The behavior can occur in solution, or in the case of ionic liquids used as nonvolatile solvents, occurs in the pure state. Electrolytes exist in biological and industrial systems and are thus important to our everyday life.
Sodium chloride almost totally dissociates into sodium and chloride ions in dilute aqueous solutions near room temperature. Due to this strong dissociation, sodium chloride is described in these circumstances with a strong electrolyte model by assuming that the dissociation is complete. On the other hand, acetic acid, the dominant ingredient in household vinegar, partially dissociates into hydrogen ions and acetate ions in dilute aqueous solution and is modeled as a weak electrolyte. A key caution is that the literature tends to describe compounds as strong or weak electrolytes. However, the extent of dissociation depends on the environment. For example, Fig. 18.1 shows the distribution of species in sulfuric acid as a function of concentration measured experimentally1 and modeled.2 Both hydrogens dissociate only in very dilute solution (which is difficult to discern from the figure). The second dissociation is suppressed at moderate and high concentrations. The first dissociation disappears at high concentrations and sulfuric acid exhibits limited dissociation when pure. Experimental information on the degree of dissociation is important as well as thermodynamic models to represent the behavior to characterize the strong or weak dissociation under the conditions of interest.
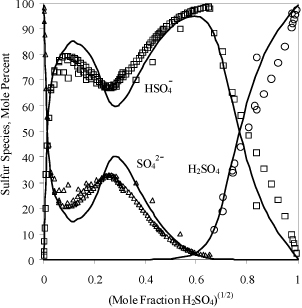
Figure 18.1. Speciation of sulfuric acid in aqueous solutions as measured by experiments1 and modeled by OLI Systems, Inc.2 Note the square root scale to emphasize the dilute region.
The extremely polar nature of water and the capability of water to adjust its partial charges by adjusting the H-O bond distance make it capable of hydrating or solvating the ions. The water surrounding the ion is known as the water of hydration or solvation and the ions are described as solvated. Fundamental studies show that three to five water molecules hydrate ions in dilute to moderately concentrated solutions. The number is inexact due to the difficulty of the experiments and the fact that the hydration shell is not forming stoichiometric bonds, so the whole hydrated ion is constantly undergoing exchange resulting in various packing effects and various sizes. The key point is to understand that ions must be solvated in solution, and the degree of electrolyte dissociation depends on the ability of a solvent to dissolve ions and the competing driving force for the electrolyte to stay undissociated. Solvents must have a high dielectric constant to be capable of solvating ions. Sodium chloride has an infinitesimal solubility in hexane because the ions are not solvated effectively.
Electrolytes can also have important effects in inhomogeneous fluids like surfaces and colloids. For example, the ions may align next to a surface of opposite charge causing a phenomenon known as an electric double layer. Ions can also strongly affect surfactants in micelles, emulsions, and microemulsions. We restrict our attention here to bulk, homogeneous systems, which are affected in relatively straightforward ways by reaction and dilution.
Leave a Reply