Olivine is a name for a series of minerals with the formula M2SiO4, where M is most commonly Fe or Mg. Fayalite (Fe2SiO4) and forsterite (Mg2SiO4) form a substitutional solid solution where the iron and magnesium atoms can be substituted for each other without significantly changing the crystal structure. As mentioned previously, there is a size mismatch of only about 7% between Mg2+ and Fe2+, so complete solid solution between these two elements is observed in olivine.
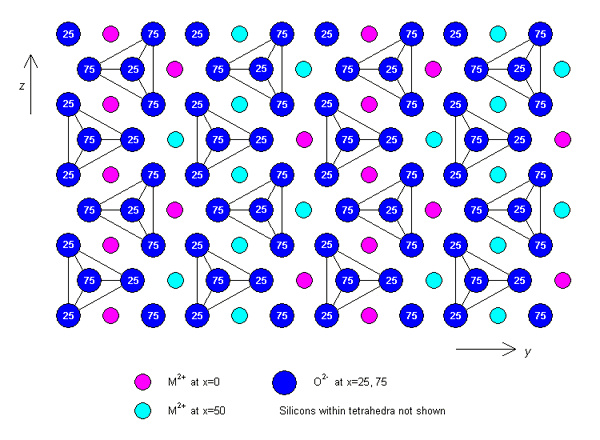
Olivine has an orthorhombic structure. A typical set of lattice parameters for an unspecified composition are: a = 0.49 nm, b = 1.04 nm, c = 0.61 nm. The structure consists of isolated SiO44- tetrahedra, which are held together by M cations occupying two types of octahedral site (M1 and M2). The isolated tetrahedra point alternately up and down along rows parallel to the z-axis.
Alternatively, the structure can be described as an approximately hexagonal close-packed array of oxygen anions, with M cations occupying half of the octahedral sites, and Si cations occupying one eighth of the tetrahedral sites. If the hexagonal close packing were ideal, the M1 and M2 sites would be regular octahedra, and identical in size, but since the packing is not ideal, the M2 sites are slightly larger and more distorted than the M1.
A plan view of the structure projected along the x-axis is shown below.
Leave a Reply