A binary solid solution can show a phase diagram as follows:
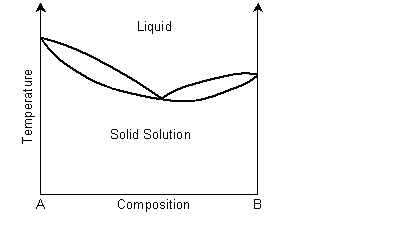
The solidus and liquidus separate the single phase regions. This is similar to the Cu-Ni phase diagram. If the system demonstrates exsolution, then there will be a region in the solid state where two solid phases form from the solid solution. This is common and most solid solutions demonstrate it to some degree.
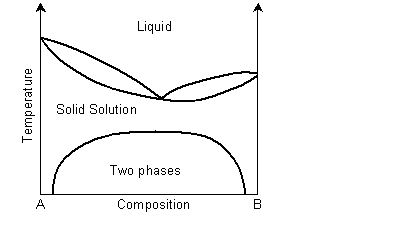
View information on exsolution in polymer mixtures.
In a eutectic phase diagram, the two-phase solid region meets the solidus/liquidus.
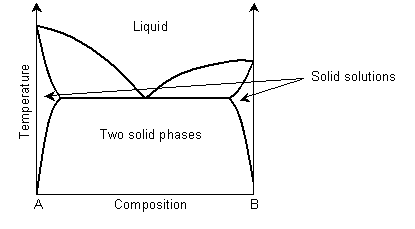
Solid solution regions are usually denoted with Greek letters, for example α for ferritic iron and γ for austenitic iron.
Leave a Reply